Subscribe
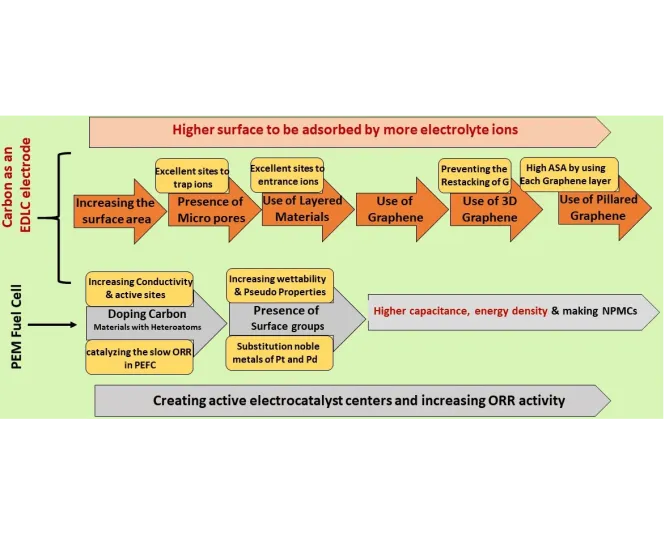
Progress in active material synthesis for EDLC and PEM Electrodes
20.09.2024
In EDLCs, increasing the surface area of the active material enhances capacitance due to their working mechanism. The flowchart above outlines the steps to achieve higher accessible surface area and capacitance. Additionally, introducing heteroatoms like N, S, P, and B into the carbon structure is an effective method to further boost capacitance. These heteroatoms can function as n/p-type dopants, improving electron mobility (conductivity) and enhancing the pseudo-capacitance properties of EDLCs. Fuel cells, on the other hand, require rare and expensive noble metals like Pt and Pd as electrocatalysts, especially for the cathode. However, heteroatom-doped carbon materials can serve as Non-Precious Metal Catalysts (NPMCs) by acting as electroactive centers for the oxygen reduction reaction (ORR) in the PEM cathode.
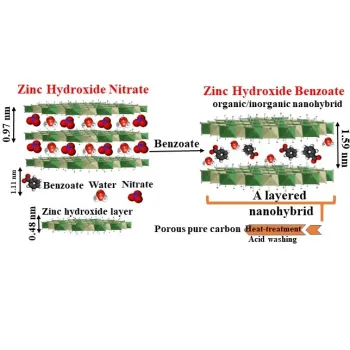
Our method: using ofα-phase metal hydroxides to produce porous carbon
20.09.2024
The thermal decomposition of layered organic-inorganic nanohybrids synthesized by the hybridization of an organic anion with a layered metal hydroxide results in the production of highly porous carbon material and metal oxide. By removing the metal oxide using an acid, pure carbon material is produced
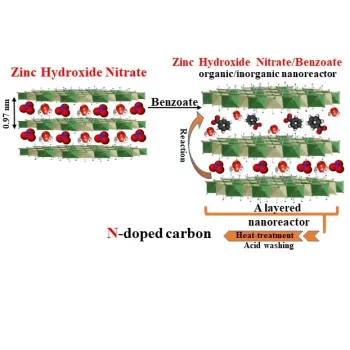
Using of α-phase metal hydroxides to produce porous doped-carbon
20.09.2024
The thermal decomposition of layered organic-inorganic nanohybrids synthesized by incomplete anion exchange, where organic ions partially replace inorganic ions between layers of metal hydroxide, results in the production of highly porous doped carbon material and metal oxide. This means that the nanohybrid contains both organic and inorganic ions (such as nitrate) between the layers. By removing the metal oxide using an acid, the doped carbon material is produced.

Our commercial-like electrodes
20.09.2024
In the manufacture of electrodes for supercapacitors and Li-ion, Na-ion, and other battery anodes, most articles describe the preparation of a paste consisting of active materials, conductive carbon black, and a binder in a mass ratio of 80:10:10, respectively. However, in actual electrodes, this ratio is often not accurate. Each active material has distinct properties, such as surface area, conductivity, and morphology, and the percentages of these three components must be carefully balanced to achieve the highest areal capacity with minimal electrical resistance in the electrodes.

Our high-voltage & efficient SC device
20.09.2024
A commercial-like symmetric SC device was fabricated by using N,S-doped carbon nanosheets and an organic commercial electrolyte. The device exhibited a high capacitance of 33 F/g, an energy density of 41 Wh/kg, and a power density of 1500 W/kg using a conventional slow charge-discharge approach. It also demonstrated a capacitance retention of over 90% after 1000 cycles in a fast charge approach. See https://pubs.acs.org/doi/10.1021/acsaem.4c01440
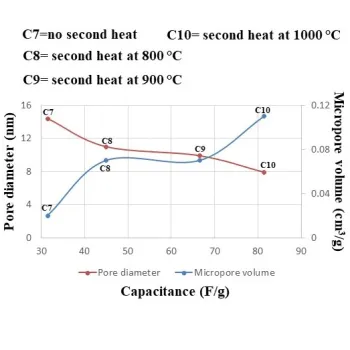
Why is a second heat treatment so important for the template synthesis of carbon materials in SC applications?
20.09.2024
We investigated the effect of a second heat-treatment on the capacitance for the synthesized carbon material. The results showed that supercapacitors exhibit a higher capacitance, although the amount of porosity and surface area decrease. Also, the surface oxygenated groups of carbon materials were removed using the second heat treatment. This in turn eliminates faradic Red-Ox reactions from the active material, but due to the increased conductivity, the capacity increases with the second heat treatment.